Home / News / India News / Article /
No Sputnik V approval yet, SEC asks Dr Reddy's for more data on vaccine
Updated On: 02 April, 2021 11:27 AM IST | New Delhi | IANS
So far, more than six crore doses of corona vaccine have been administered in the country since the drive began on January 16 after the approval for 'Covishield' and 'Covaxin'.
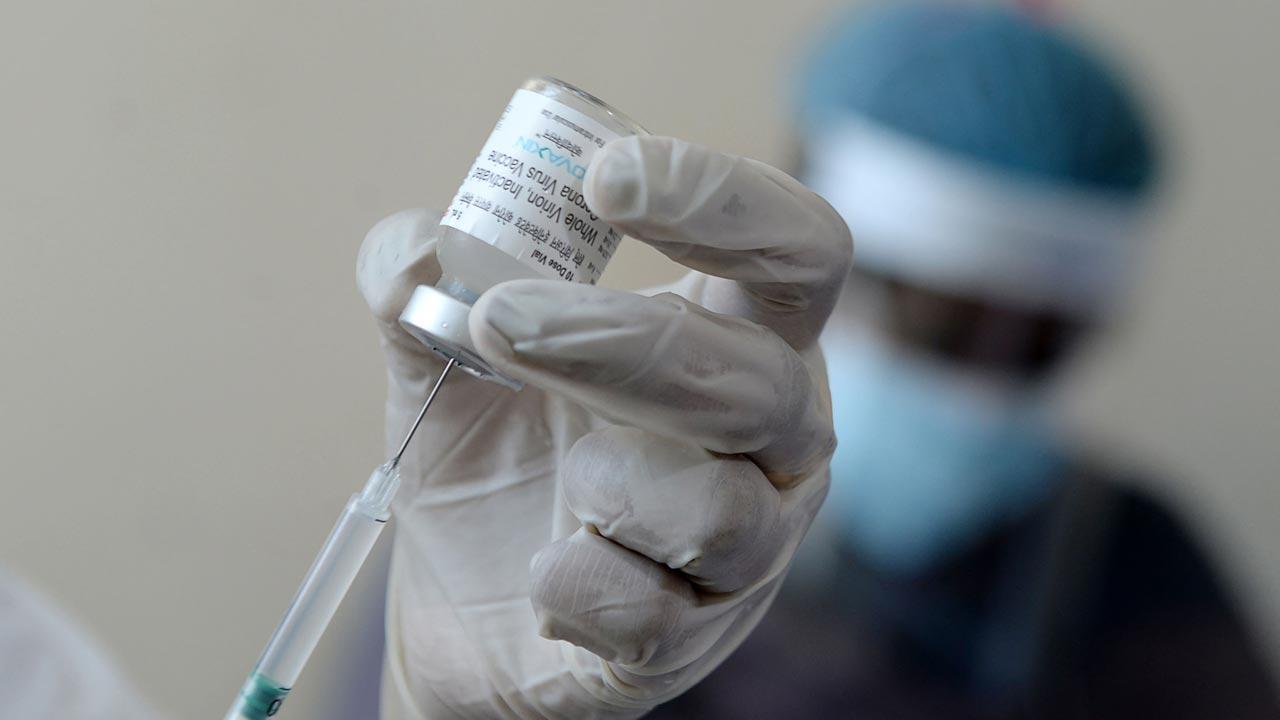
A medical worker prepares to inoculate a person with the Covaxin, at a government hospital as India expanded its coronavirus vaccination drive to the 45-60 age group. Pic/PTI
The Subject Expert Committee (SEC) of the Indian drug regulator, tasked with vetting Covid-19 vaccine proposals, sought additional data on Russian Covid-19 vaccine 'Sputnik V', while deciding on its emergency use authorisation, a senior government official said on Thursday.
In September 2020, Dr. Reddy's had partnered with the Russian Direct Investment Fund (RDIF) to conduct the clinical trials of Sputnik V and for its distribution rights in India. The company had conducted Phase 2 trials in India with 1,500 participants. Phase three trials are underway.



