Home / News / India News / Article /
Sputnik V could be India's 3rd COVID-19 vaccine: Experts
Updated On: 05 March, 2021 07:43 AM IST | New Delhi | IANS
Sputnik V has demonstrated an efficacy rate of 91.6 per cent in the interim analysis of the Phase 3 clinical trials, which included data on 19,866 volunteers in Russia, who received both the first and second dose of the vaccine.
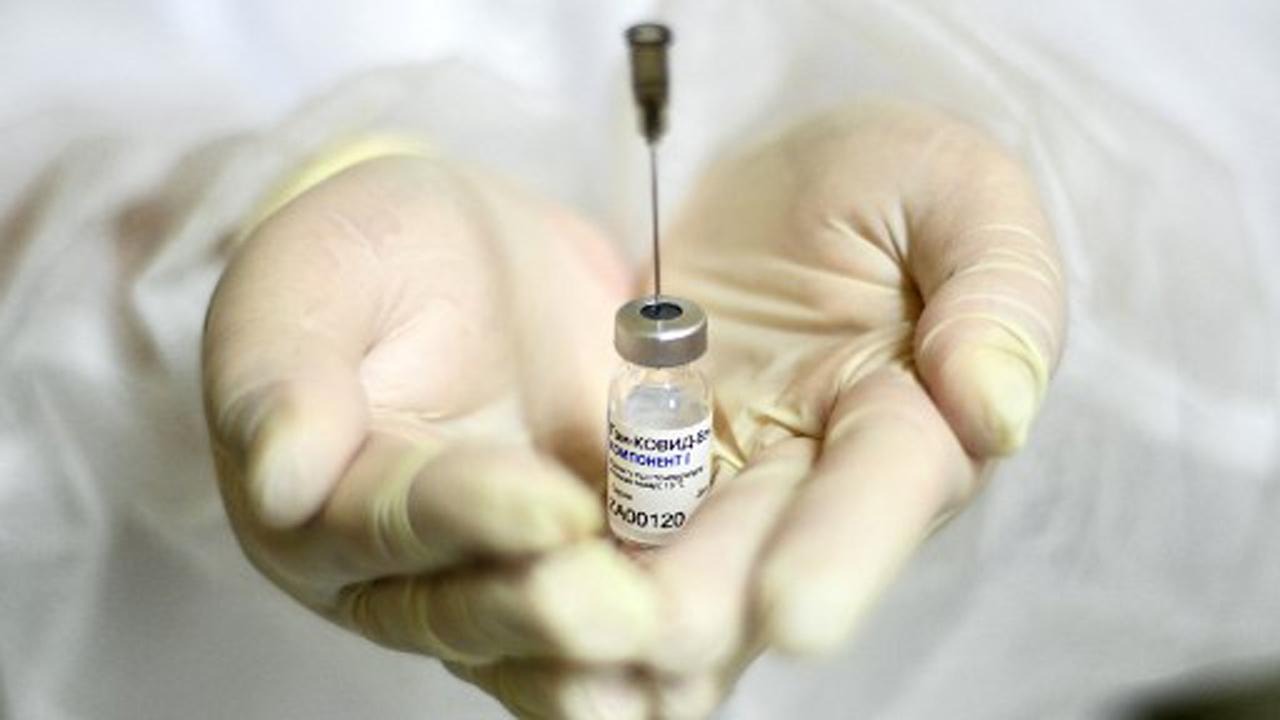
In this file photo taken on December 5, 2020, a nurse shows the Sputnik V (Gam-COVID-Vac) vaccine against the coronavirus disease (COVID-19) at a clinic in Moscow amid the ongoing coronavirus disease pandemic. Pic/AFP
To cater to the vaccination process of the world's largest democracy, it would be pertinent to bring in safer and more effective vaccines from other countries.
However, on February 24, India's drug authority had asked Dr Reddy's, the Indian pharmaceutical company facilitating the trials in the country, to supply data on immunogenicity for going ahead with the approval process. However, experts across the country have been questioning the approval procedure given the fact that the country gave approval to two homegrown vaccines in January this year for emergency use based on modified standards. For instance, Covaxin was granted restricted approval in "clinical trial mode" without any efficacy data.



