Home / News / World News / Article /
Alzheimer’s drug that can slow disease gets backing from FDA
Updated On: 12 June, 2024 07:45 AM IST | Washington | Agencies
The FDA approved a similar infused drug, Leqembi, from Japanese drugmaker Eisai last year
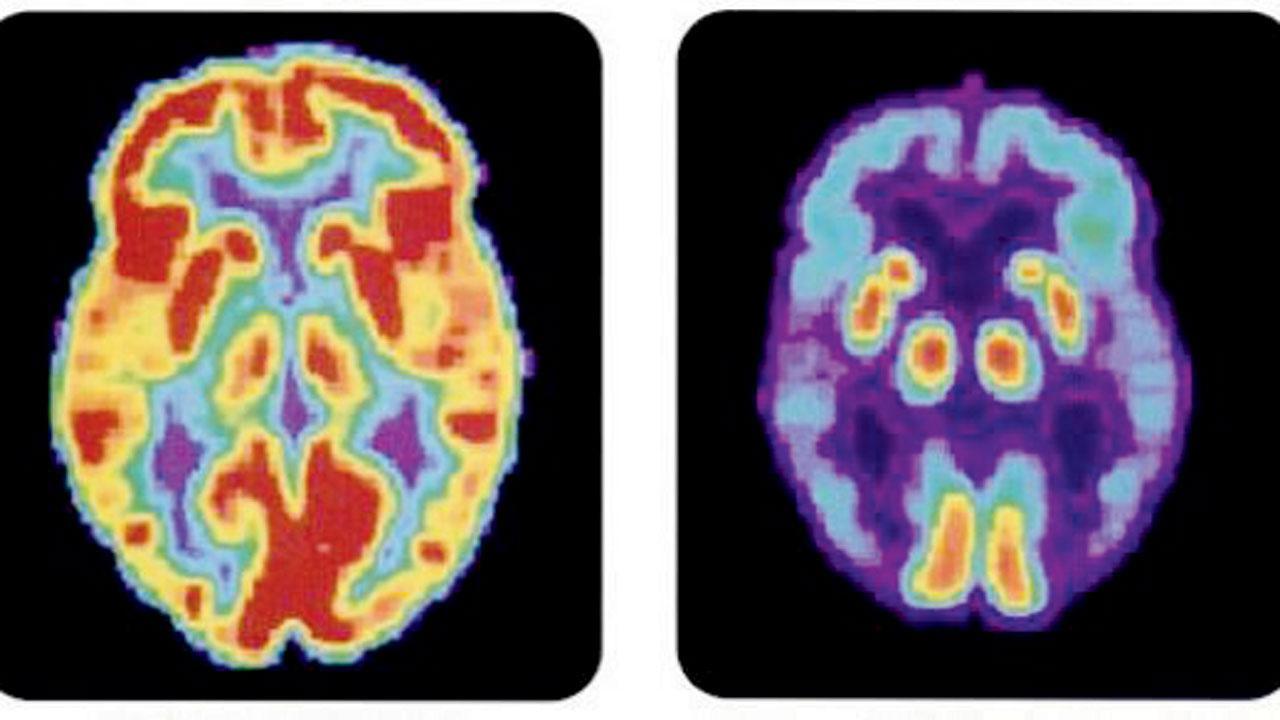
PET scan of normal brain vs brain affected by Alzheimer’s disease
A closely watched Alzheimer’s drug from Eli Lilly won the backing of federal health advisers on Monday, setting the stage for the treatment’s expected approval for people with mild dementia caused by the disease.
A panel of Food and Drug Administration advisers voted unanimously that the drug’s ability to modestly slow the disease outweighs its risks, including side effects like brain swelling and bleeding that will have to be monitored. “I thought the evidence was very strong in the trial showing the effectiveness of the drug,” said panel member Dean Follmann from the National Institutes of Health.



