Home / News / World News / Article /
Moderna begins testing COVID-19 vaccine on children
Updated On: 16 March, 2021 12:00 AM IST | New York | IANS
This Phase 2/3 two-part and randomised study will evaluate the safety, tolerability, reactogenicity and effectiveness of two doses of mRNA-1273 given 28 days apart.
Listen to this article :
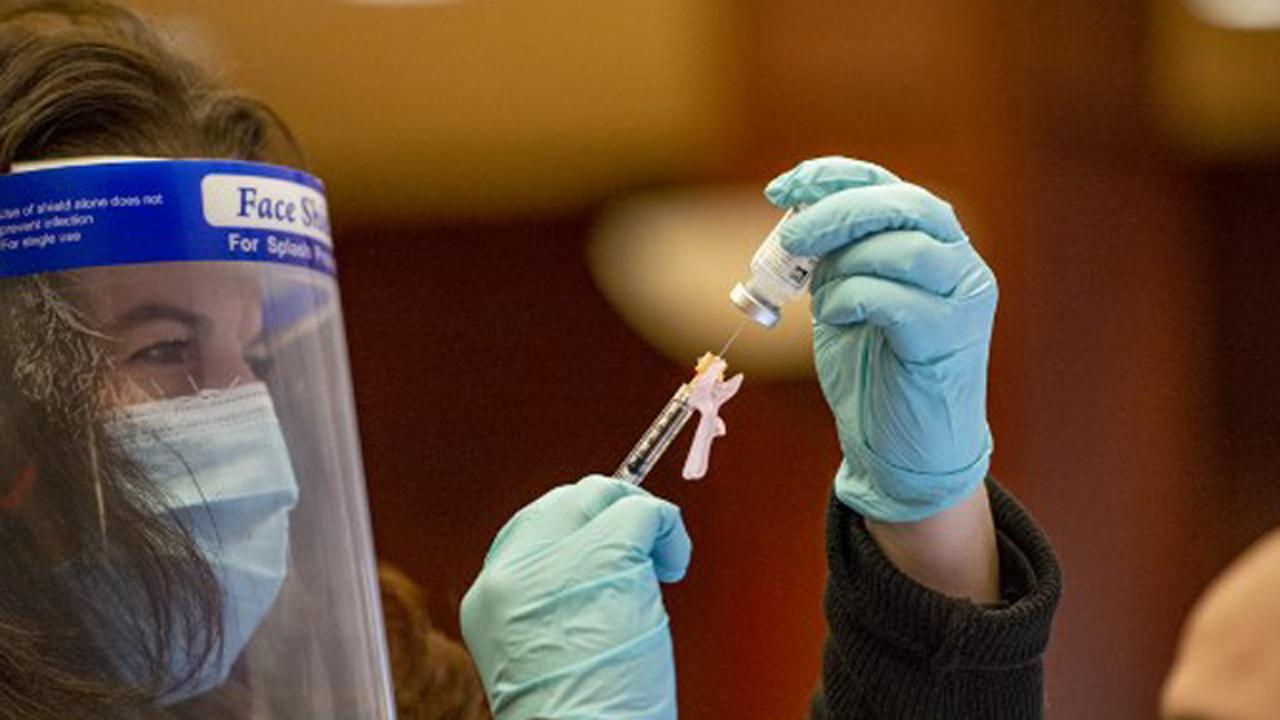
Nurse Laura Bailey draws the Moderna vaccine from the first batch of Moderna`s vaccine at Hartford hospital in Hartford, Connecticut on December 21, 2020. Pic/AFP
US-based Biotechnology company Moderna on Tuesday announced that it is now testing a Covid-19 vaccine designed to be used on children aged between 6 months to less than 12 years old.
To begin with, the company intends to enrol approximately 6,750 paediatric participants in the US and Canada.



