Home / News / World News / Article /
Moderna begins testing COVID-19 vaccine on children
Updated On: 17 March, 2021 10:46 AM IST | New York | IANS
To begin with, the company intends to enrol approximately 6,750 paediatric participants in the US and Canada.
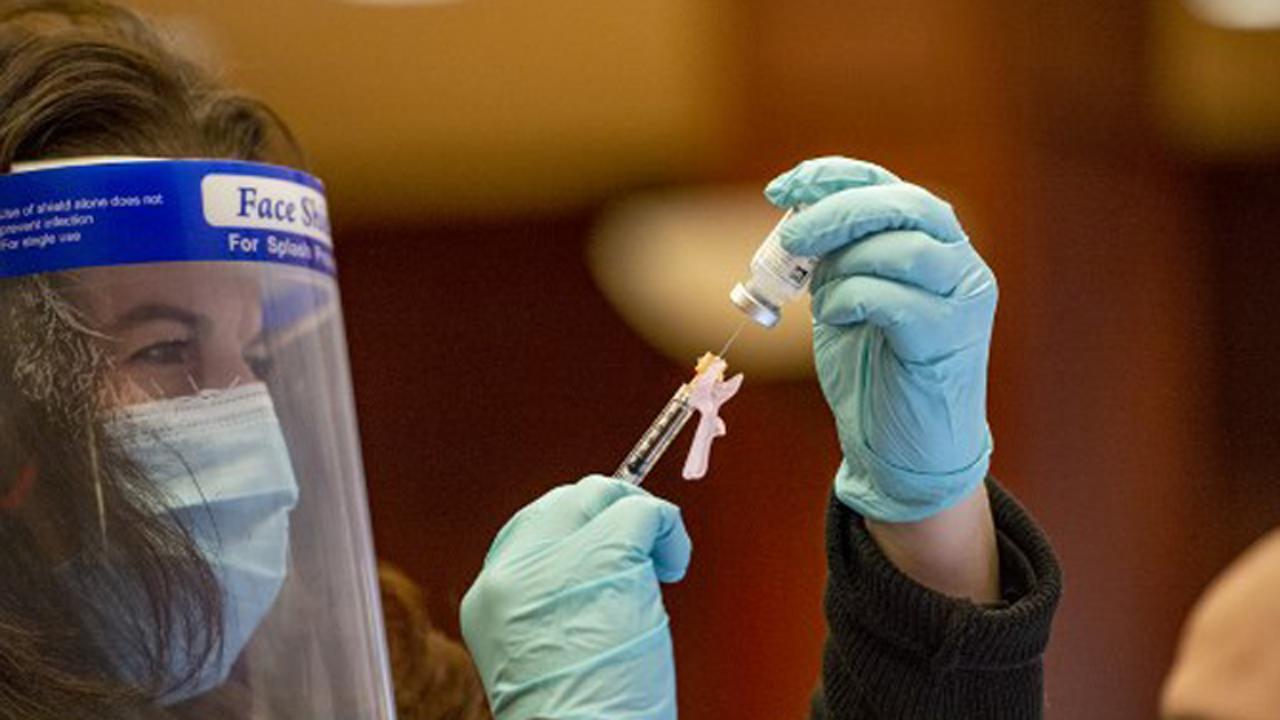
Nurse Laura Bailey draws the Moderna vaccine from the first batch of Moderna`s vaccine at Hartford hospital in Hartford, Connecticut. Pic/AFP
US-based Biotechnology company Moderna on Tuesday announced that it is now testing a Covid-19 vaccine designed to be used on children aged between 6 months to less than 12 years old. To begin with, the company intends to enrol approximately 6,750 paediatric participants in the US and Canada. The trials are being conducted in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and the Biomedical Advanced Research and Development Authority (BARDA).
"We are pleased to begin this Phase 2/3 study of mRNA-1273 in healthy children in the US and Canada and we thank NIAID and BARDA for their collaboration," Stephane Bancel, Chief Executive Officer of Moderna, said in a statement. "We are encouraged by the primary analysis of the Phase 3 COVE study of mRNA-1273 in adults ages 18 and above and this paediatric study will help us assess the potential safety and immunogenicity of our Covid-19 vaccine candidate in this important younger age population," Bancel added.



